Description
(MDA APPROVED) VITAVUE 10 – IBP Touch Screen
MDA Registration No. GC4126925-211702
Vitavue Series patient monitor:
– portable and easy-to-use multi-parameter patient monitoring device with reliable performance
– small size, light and practical and combined with a multi-parameter monitoring function (can realize real-time).
– comprehensive monitoring of patients.
– Facilitating the review and analysis of data by healthcare professionals.
Function Module:
– ECG, 3/5 Lead
– SpO2 (S5)
– NIBP (N5)
– IBP
– TEMP .x2
– Touch Panel
Inclusive Accessories:
– SpO2 patient cable with reusable finger sensor (Adult)
– NIBP Tubing (Hose)
– NIBP Cuff (Adult)
– Skin Temperature probe
– ECG 5 Lead Cable
– ECG Electrodes
– IBP Cable
– Pressure Transducer
Warranty:
1 Year Warranty Against Manufacturing Defects for the Main Device Only. No Warranty for Accessories.



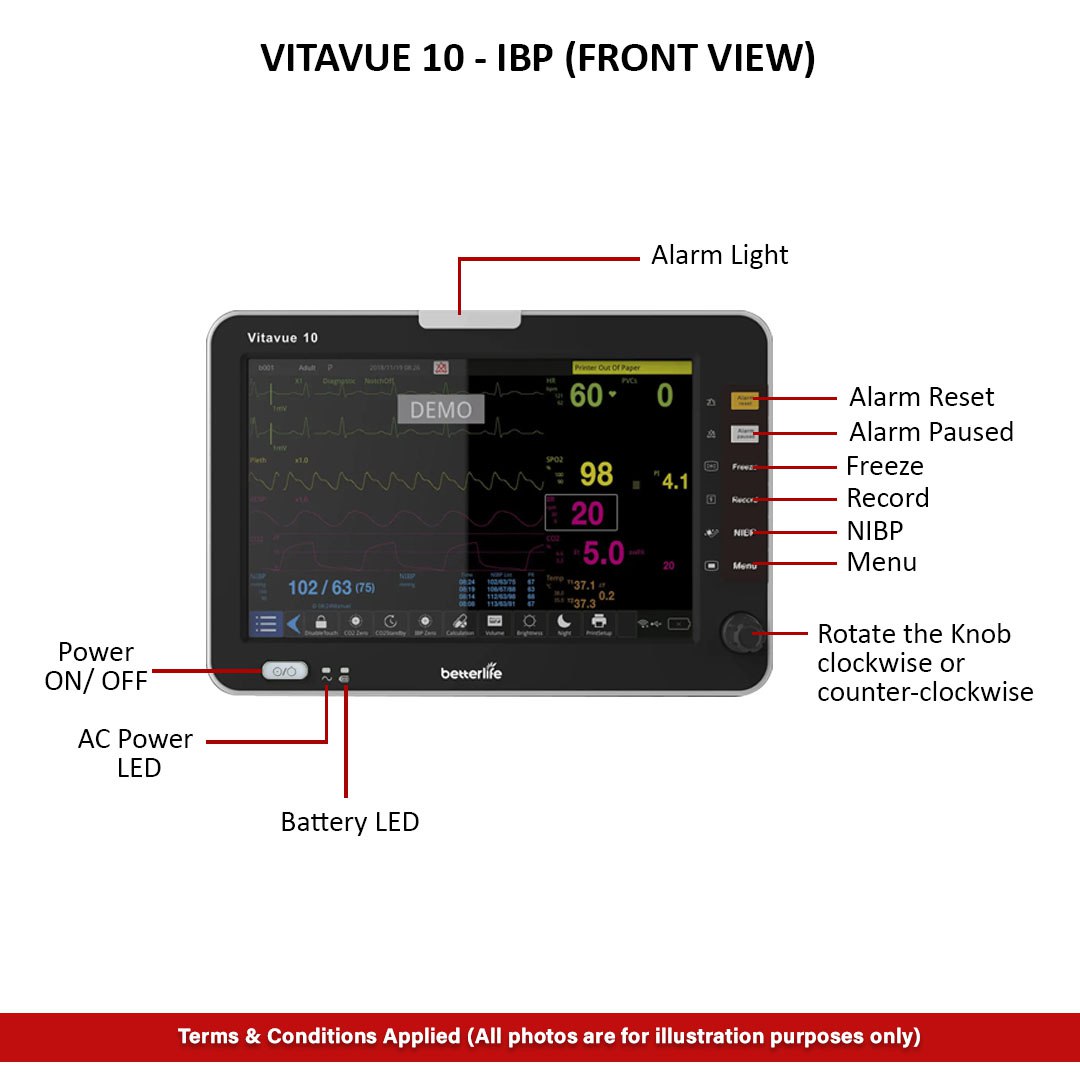
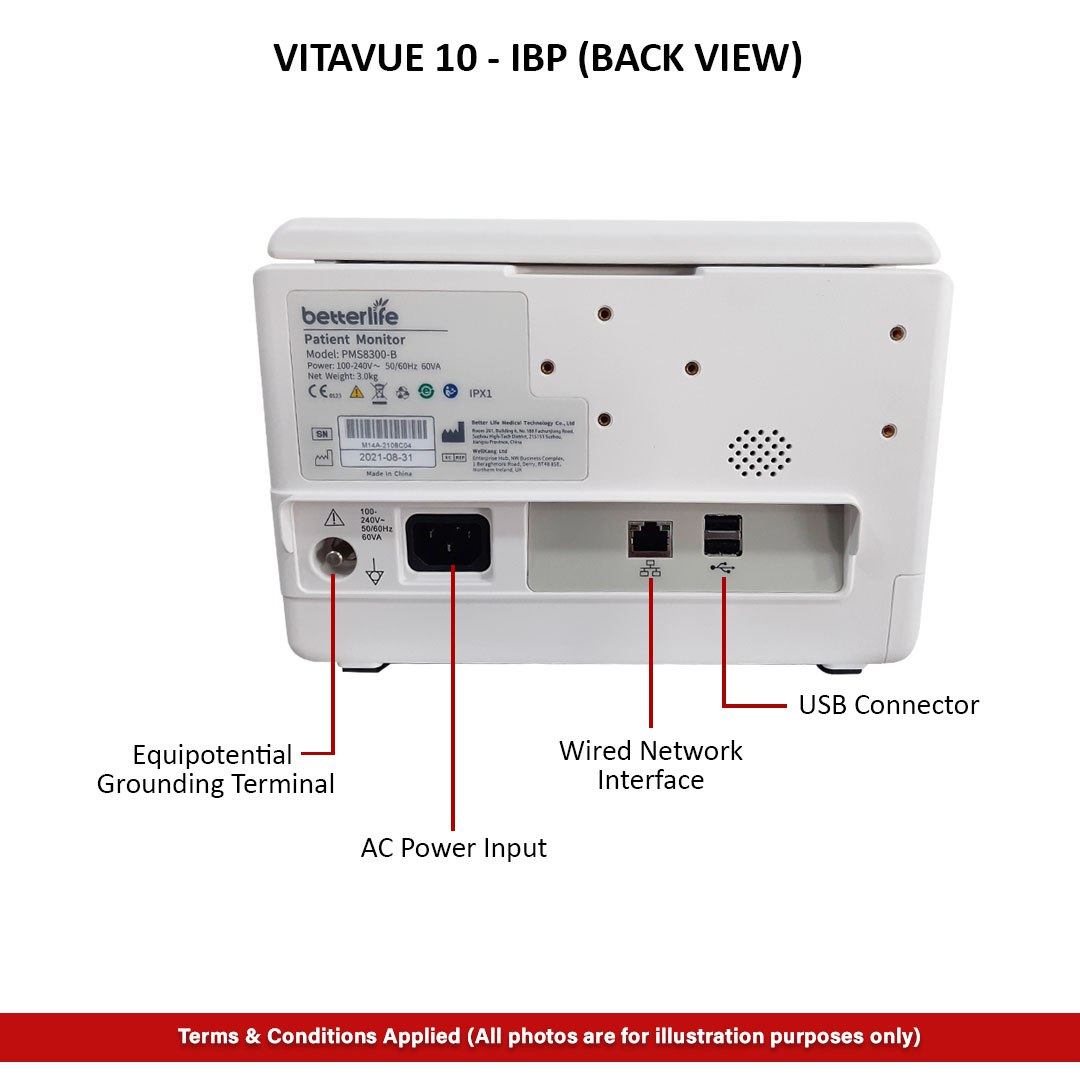
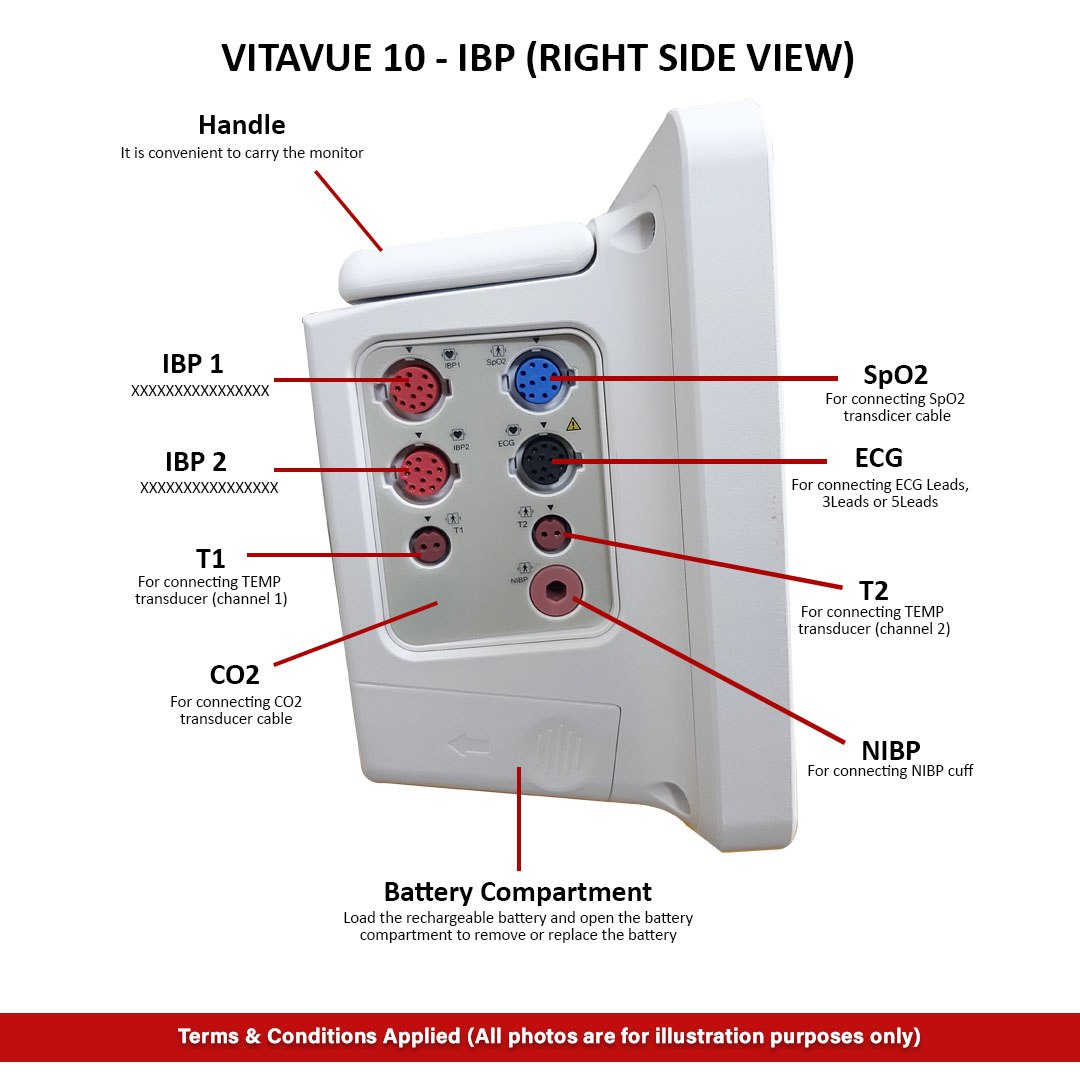




Reviews
There are no reviews yet.